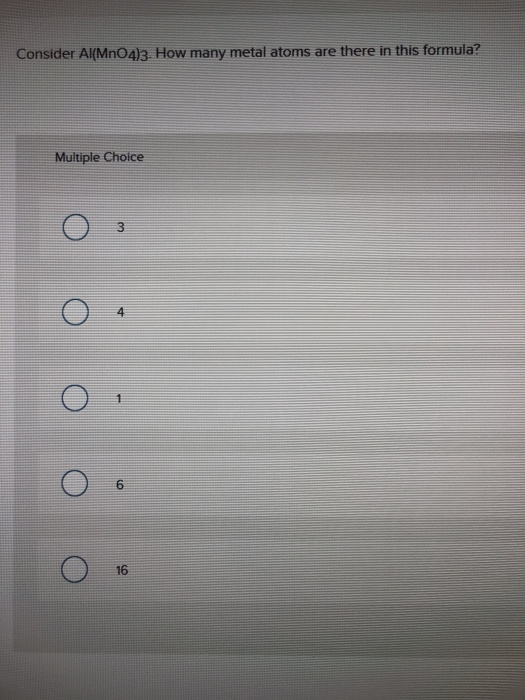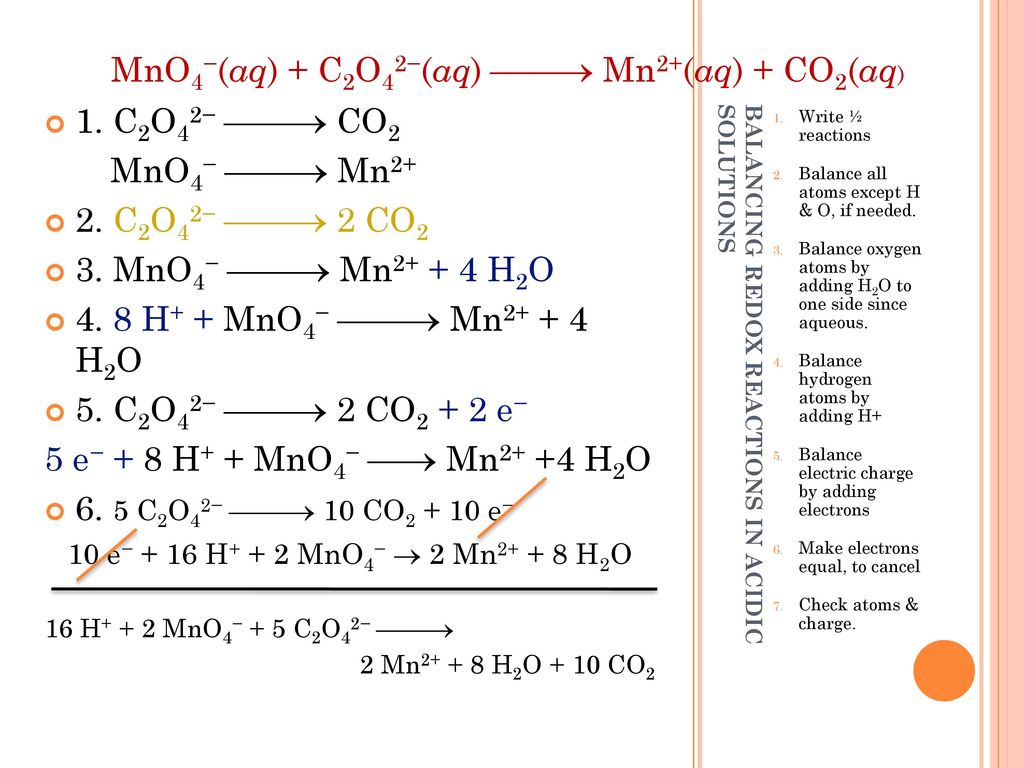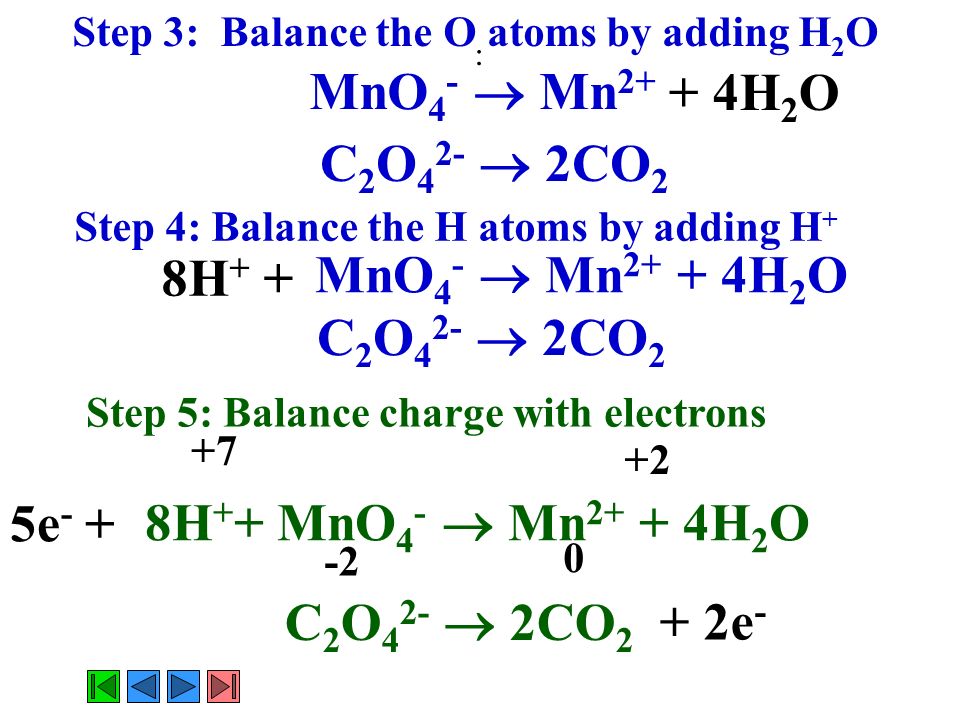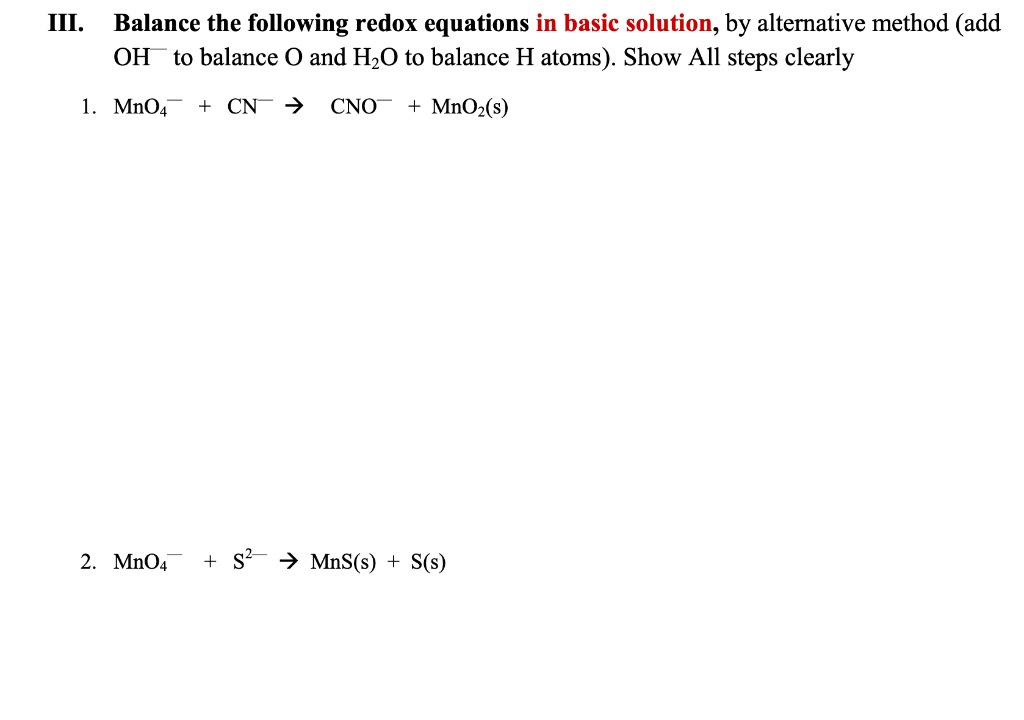
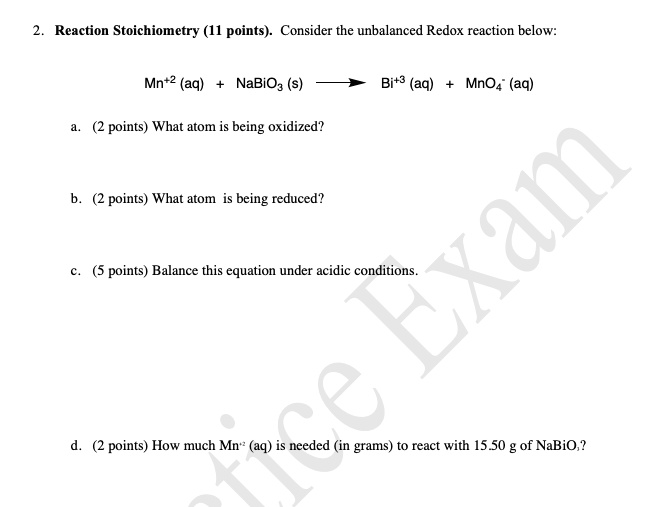
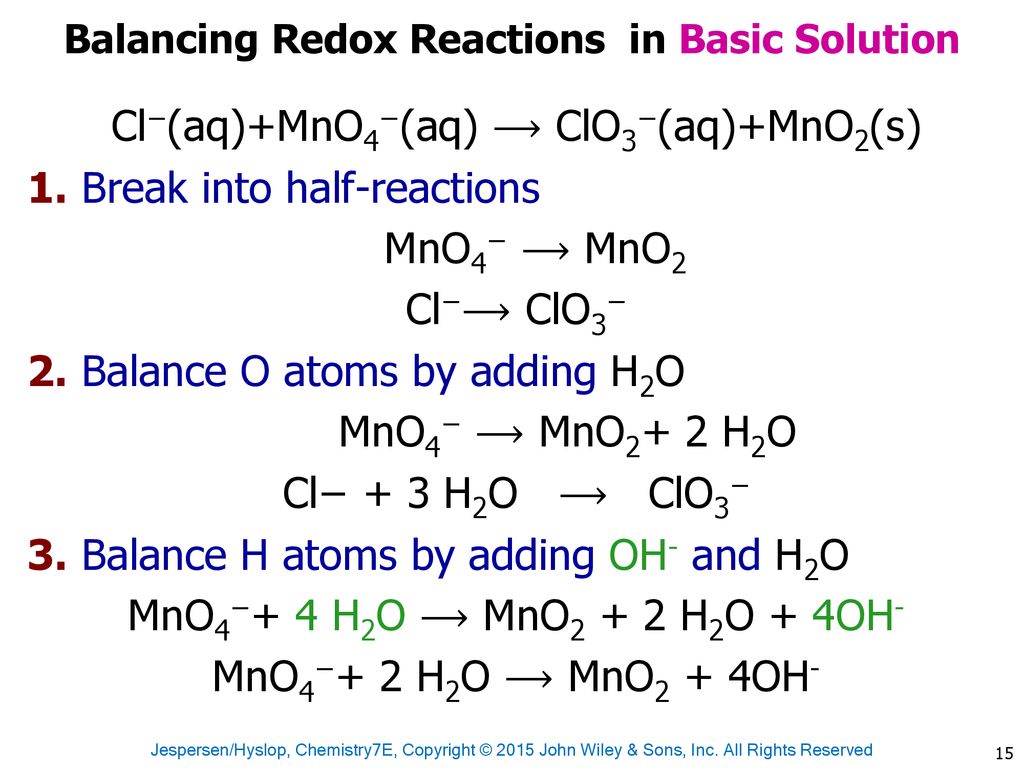
SOLVED:III: Balance the following redox equations in basic solution, by alternative method (add OH to balance 0 and HzO to balance H atoms). Show All steps clearly MnO4 CN 7 CNO MnOz(s)

If S + O2→ SO2, H = - 298.2 kJ mole^-1 SO2 + 1/2 O2→ SO3, H = - 98.7 kJ mole^-1 SO3 + H2O → H2SO4, H = - 130.2 kJ

MnO4 and PO4 tetrahedra in Mn3(PO4)4‚2(N4C6H21)‚ 6(H2O): MnO4 open,... | Download Scientific Diagram

.PNG)









.PNG)